Coin CdN 2012-1-57
Rey-Bellet. Bernadette (, None) & Naima. Gutknecht (HE-Arc CR, Neuchâtel, Neuchâtel, Switzerland) & Valentina. Valbi (Laboratoire Métallurgie et Culture LMC-IRAMAT-CNRS-UTBM, Belfort, Franche-Comté, France)
Coin with blue/green and localised white corrosion products. The coin is probably an imitation. Diameter: about 1.2cm.
coin
Peney, Genève, Geneva, Switzerland
1960
Late roman times
Soil
Musée d'art et d'histoire, Genève, Geneva
Musée d'art et d'histoire, Genève, Geneva
CdN 2012-1-57
No conservation treatement reported
Object recovered in 1960 at Peney, Geneva, Switzerland in a hoard containing 4000 coins. Around 1400 coins from the hoard are conserved at the Art and History Museum of Geneva.
The schematic representation below gives an overview of the corrosion structure encountered on the coin from a first visual macroscopic observation.
| Stratum | Type of stratum | Principal characteristics |
| CP1 | Corrosion product | Cluster, light yellow, thin, scattered, compact, powdery, very soft |
| CP2 | Corrosion product | Lens-shaped, light brown, thick, isolated, non-compact, powdery, soft |
| CP3 | Corrosion product | Cluster, blue, medium, scattered, compact, brittle, soft |
| CP4 | Corrosion product | Cluster, dark green, medium, scattered, compact, severable, soft |
| M1 | Metal | Light grey, metallic, continuous, compact, tough, soft |
Table 1: Description of the principal characteristics of the strata as observed under binocular microscope according to Bertholon's method.
The cross-section corresponds to a cut of the coin in half (Fig. 2) and is representative of the entire thickness of the coin's body. A metallic core is present below the corrosion layers (Fig. 7).
Cu alloy
Cast and cold worked (with final annealing?)
BC57
Musée d'art et d'histoire, Genève, Geneva
Musée d'art et d'histoire, Genève, Geneva
June 2021
None.
Analyses performed:
Non-invasive approach
- XRF with handheld portable X-ray fluorescence spectrometer (NITON XL3t 950 Air GOLDD+, Thermo Fischer®). General Metal mode, acquisition time 60s (filters: Li20/Lo20/M20).
Invasive approach (on the sample)
- Optical microscopy: the sample is polished, then it is observed on a numerical microscope KEYENCE VHX-7000 in bright and dark field.
- Metallography: the polished sample is etched with alcoholic ferric chloride and observed by optical microscopy in bright field.
- SEM-EDS: the sample is coated with a carbon layer and analyses are performed on a SEM-FEG JEOL 7001-F equipped with a silicon-drift EDS Oxford detector (Aztec analysis software) with an accelerating voltage of 20 kV and probe current at about 9 nA. The relative error is considered of about 10% for content range <1mass%, and of 2% for content range of >1mass%.
- µ-Raman spectroscopy: it is performed on a HORIBA Labram Xplora spectrometer equipped with a 532 nm laser with 1800 grating, the laser power employed is between 0.04 and 0.55 mW with acquisition time varying between 1 and 5 minutes.
The XRF analysis of coin CdN 2012-1-57 was carried out before sampling. All strata, from soil and corrosion products to metal, are analyzed at the same time. The metal is presumably a copper-lead-tin alloy, while the other elements detected (Fe, Si, Al) are from the environment.
| Elements | mass % |
| Cu | 46 |
| Pb | 46 |
| Sn | 3 |
| Si | 3 |
| Fe | <1 |
| Al | 3 |
Table 2: Chemical composition of the surface of coin CdN 2012-1-57. Method of analysis: XRF, General Metal mode, acquisition time 60s (filters: Li20/LO20/M20). The results are rounded up to the nearest whole number, UR-Arc CR.
The central area of the sample is totally corroded.
EDX analysis (Table 3) of the residual metal on cross-section indicates that it is a Cu-Pb-Sn ternary alloy with a high percentage of Pb (25 wt%). This confirms XRF analysis (Table 2).
| Elements | wt% |
| Cu | 69 |
| Pb | 26 |
| Sn | 5 |
Table 3: Chemical composition (wt%) of the alloy over a general area of analysis, LMC-IRAMAT-CNRS-UTBM.
The sample presents on its whole thickness big Pb inclusions (50-200 μm, Figs. 8) homogeneously distributed. The metal has a heterogeneous microstructure. The microstructure is mostly dendritic with slip lines (Figs. 9-10) lines revealing that the object underwent cold working (Fig.9). Some areas show the presence of grain boundaries of polygonal grains (Fig.10) with slip lines, showing that the object also underwent local annealing, followed by cold working.
 Credit
LMC-CNRS, V. Valbi.
Credit
LMC-CNRS, V. Valbi.
Dendritic structure & limited grain structure (with twin lines)
Cu
Sn, Pb
None.
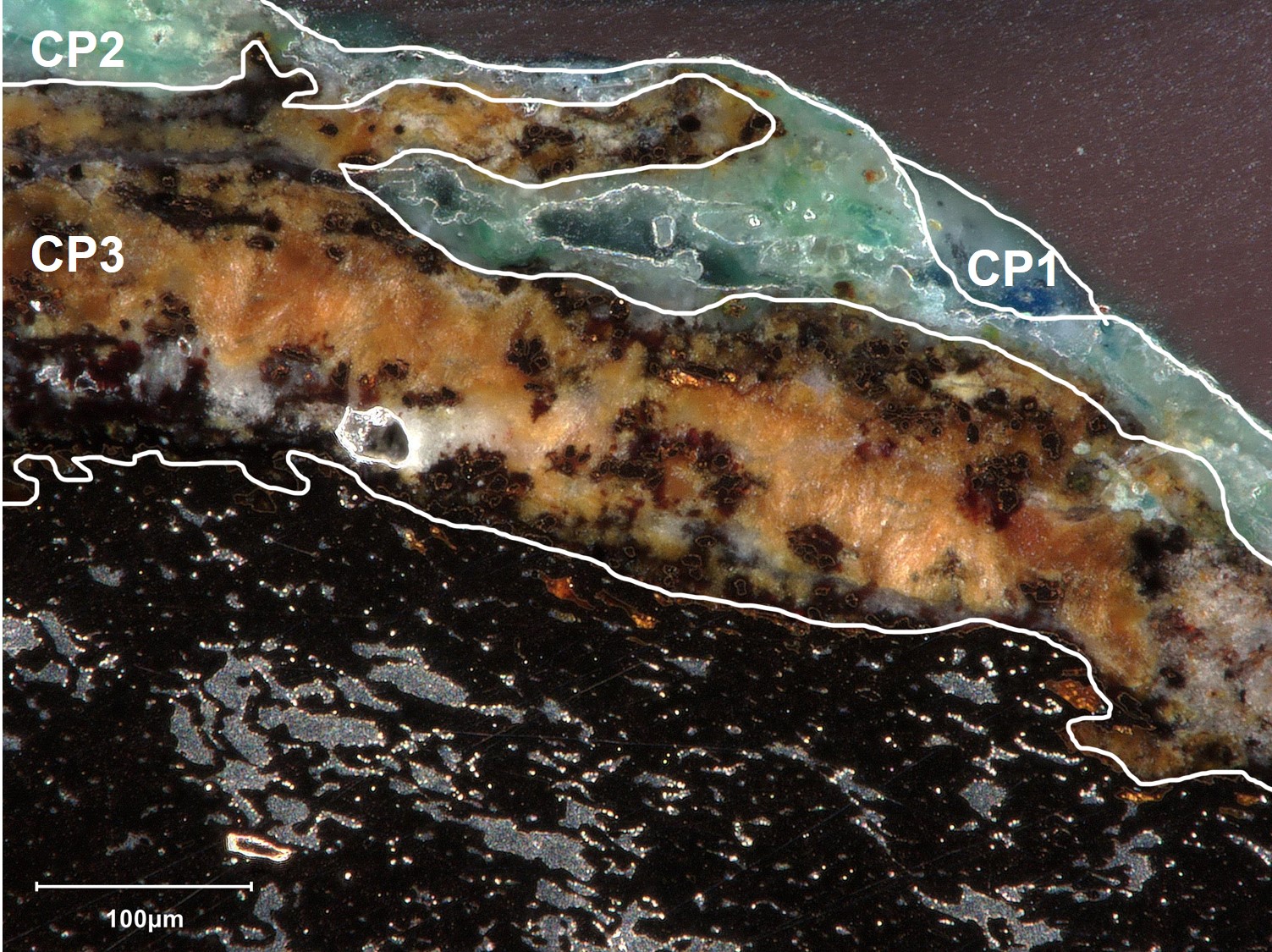
The observation of the sample in dark field mode (Fig.11) revealed the presence of an external discontinuous blue CP1 layer, a white/greenish CP2 layer and a thick internal orange CP3 layer.
The EDX elemental analysis (Table 4, Fig.12) of the visually identified CPs by cross-sectional observation shows that the external CP1 is a Cu-based compound, while the white CP2 and the orange CP3 are lead-based corrosion products. Local Cl enrichments (3 wt%) and Cu uncorroded areas are observed in CP3.
µ-Raman analyses were performed on the identified strata (Fig.13). The Raman spectrum obtained on the blue CP1 layer corresponds to the one of azurite (Cu3(CO3)2(OH)2). The Raman spectrum obtained on the white CP2 corresponds to cerussite (PbCO3), while the orange CP3 layer corresponds to litharge (PbO).
The central area of the sample, which is totally corroded, shows the same stratigraphy described here and is mainly composed of litharge with small areas of uncorroded copper (CP3).
| Elements | CP2 | CP3 |
| O | 20 | 7 |
| Cu | 3 | 1 |
| Pb | 77 | 92 |
Table 4: Chemical composition (wt %) of the corrosion layers over a general area of analysis in cross-section, LMC-IRAMAT-CNRS-UTBM. Carbon is not quantified by SEM-EDX because the sample was carbon-coated.
 Credit
LMC-CNRS, V. Valbi.
Credit
LMC-CNRS, V. Valbi.
Uniform
None
None.
The stratigraphies obtained by binocular and cross-section observation show a good matching (Fig. 15).
The blue CP1 observed by binocular corresponds to the blue azurite CP1 observed by CS. The green CP2 observed by binocular is also present in the CS stratigraphy. The light brown CP3 observed by binocular corresponds to the orange CP3 in CS. In CS it was possible to differentiate the carbonate cerussite and the oxide litharge.
The coin is composed of a Cu-Pb-Sn ternary bronze alloy with high Pb percentages (26 wt%). Pb is not soluble in the bronze metallic structure and high lead inclusions are observed throughout the whole metallic sample. The metallic microstructure reveals that the object has been cast and underwent cold working after local annealing (not well completed).
The external thin discontinuous corrosion products are composed of Cu-based hydroxycarbonate (azurite and probably malachite) formed by redeposition of copper with the carbonate ions from the burial environment. Lead corrosion products (litharge and cerussite) are observed as internal CPs. The presence of uncorroded Cu in the litharge layer shows that Pb is preferentially attacked. The central area of the sample, which is totally corroded, is also made of litharge with local areas of uncorroded copper. This shows that the central area of the sample had a similar composition as the rest of the sample (Cu-Pb-Sn ternary bronze). However, lead corrosion was accentuated in the central area so it is possible that this central area had a different Cu/Pb ratio from the beginning, but it is impossible to say if it was intentional or not.
This coin is part of a corpus of coins found in the same site and called "Peney Treasury". Two more coins were studied and have a MiCorr artefact sheet.
References on object and sample
1. MiCorr_Coin (white corrosion) CdN 2012-1-50
2. MiCorr_Coin (blue corrosion) CdN 2012-1-55
References on analytical methods and interpretation
3. Lafuente, B., Downs, R. T., Yang, H., Stone, N. (2015) The power of databases: the RRUFF project. In: Highlights in Mineralogical Crystallography, T. Armbruster and R. M. Danisi, eds. Berlin, Germany, W. De Gruyter, 1-30.
4. Scott, D. (2006) Metallography and microstructure of ancient and historic metals. J Paul Getty Museum Publications.
5. Švadlena J., Prošek T., Strachotová KC., Kouřil M. (2020). Chemical Removal of Lead Corrosion Products. Materials, 12 (24), 5672.
6. Quaranta M., Catelli E., Prati S., Sciutto G., Mazzeo R. (2014) Chinese archaeological artefacts: Microstructure and corrosion behaviour of high-leaded bronzes. Journal of Cultural Heritage, 15 (3), 283-291.